If the pharmaceutical landscape of 2025 was defined by resilience, 2026 is defined by precision. The days of the “one-size-fits-all” blockbuster drug are fading, replaced by a new era of personalized medicine, AI-driven discovery, and complex biologic modalities.
For a biotech startup, this shift is exhilarating but perilous. The science has never been faster, but the regulatory pathways have never been more intricate. Today, developing a new therapy isn’t just about chemistry; it is about architecture. You need a blueprint that integrates drug delivery product development, regulatory foresight, and commercial viability from Day One.
This is where drug development consulting bridges the gap. We at DES Pharma are not just experts at pharma consulting; we are the specialized “architects” and “builders” who help you turn a promising molecule into a life-saving reality. In this ultimate guide, we explore the critical role of specialized consultants in the 2026 ecosystem, from early nonclinical strategy to global commercialization.
The Architects: Drug Development and Regulatory Strategy Consulting
A drug is only as good as its path to approval. In 2026, regulatory agencies like the FDA and EMA are adapting to new technologies—from digital twins in manufacturing to decentralized clinical trials.
Strategic Planning for Complex Modalities
For companies focusing on biologic drug development consulting services, the strategy must be bespoke. Unlike small molecules, biologics (including cell and gene therapies) require flexible CMC (Chemistry, Manufacturing, and Controls) strategies that can evolve with the product.
- The 2026 Shift: The FDA has recently emphasized flexible requirements for cell and gene therapies to advance innovation. Consultants help you navigate these fluid guidelines to avoid costly clinical holds.
- Pediatric Considerations: Regulatory bodies are increasingly mandating early pediatric study plans. Pediatric drug development consulting is no longer an afterthought—it is a requirement. You must design trials that address the unique ethical and physiological needs of children while satisfying the rigorous standards of the FDA’s Drug Development Process.
Nonclinical Strategy
Before a drug ever touches a patient, it must survive the gauntlet of toxicology and safety pharmacology. Nonclinical drug development consulting services are essential for designing GLP-compliant studies that answer the specific safety questions regulators will ask.
- Expert Insight: We design the “bridge” between the lab bench and the clinic, ensuring your IND (Investigational New Drug) application is robust and “first-time-right.”
The Builders: Drug Delivery Product Design and Development
In 2026, the molecule is only half the battle. How it gets into the body—the drug delivery product development—is often the differentiator between a commercial failure and a market leader.
Innovating Beyond the Pill
Patients today demand convenience. We are seeing a massive shift towards patient-centric designs, such as auto-injectors for biologics or long-acting implants that replace daily pills.
- The Role of Consultants: Drug delivery product design and development consultants work at the intersection of engineering and biology. They ensure that your device is not only user-friendly but also manufacturable at scale.
- Technical Mastery: This requires deep expertise in materials science and industrial pharmacy. Institutions like Saint Joseph’s University are at the forefront of training the next generation of scientists in these precise industrial pharmacy skills, highlighting the academic rigor required to execute these designs in a regulated environment.
The Process: Phases of Drug Development Consulting
A consultant’s value evolves as your program matures. Here is how we support you through the phases of drug development consulting:
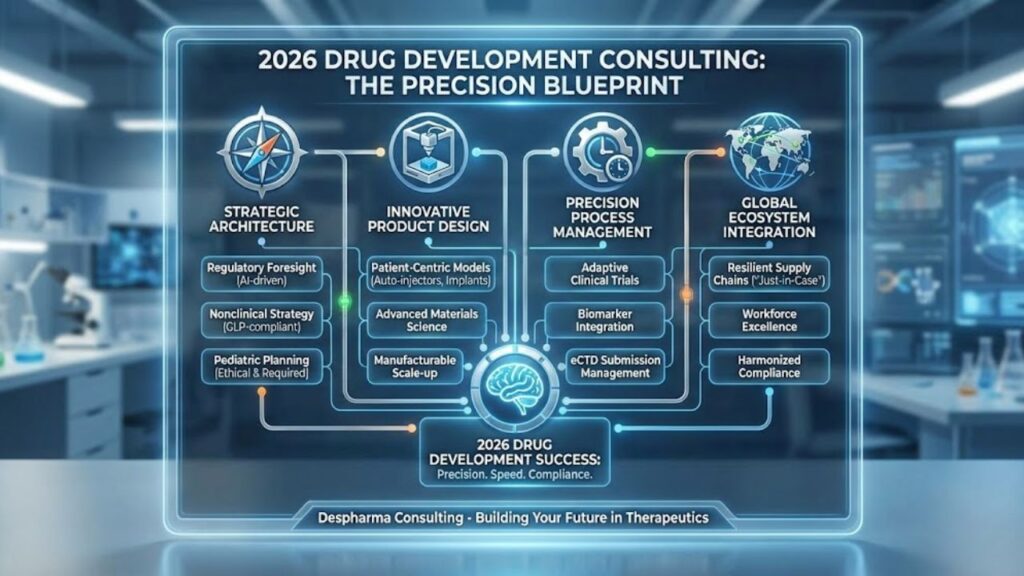
Phase 1: The Blueprint (Discovery to Preclinical)
- Focus: Target Product Profile (TPP) creation and feasibility assessments.
- Action: We conduct gap analyses to identify the missing data you need for a successful pre-IND meeting.
Phase 2: The Construction (Clinical Development)
- Focus: Clinical drug development consulting services.
- Action: Designing adaptive clinical trials that save time and money. The use of biomarkers and precision endpoints is critical here. Leading research centers, such as the Program in Regulatory Science at Dana-Farber, are pioneering new trial designs that align scientific innovation with regulatory decision-making—principles that expert consultants integrate into your specific program.
Phase 3: The Inspection (Registration & Approval)
- Focus: NDA/BLA Submission.
- Action: We act as your “regulatory gatekeepers,” compiling the massive data sets into eCTD format and managing agency interactions to ensure clarity and compliance.
The Global Ecosystem: Global Drug Development Consulting
In 2026, drug development is borderless. A molecule might be discovered in Boston, manufactured in Ireland, and tested in Australia. Global drug development consulting is about harmonizing these disparate elements into a single, compliant supply chain.
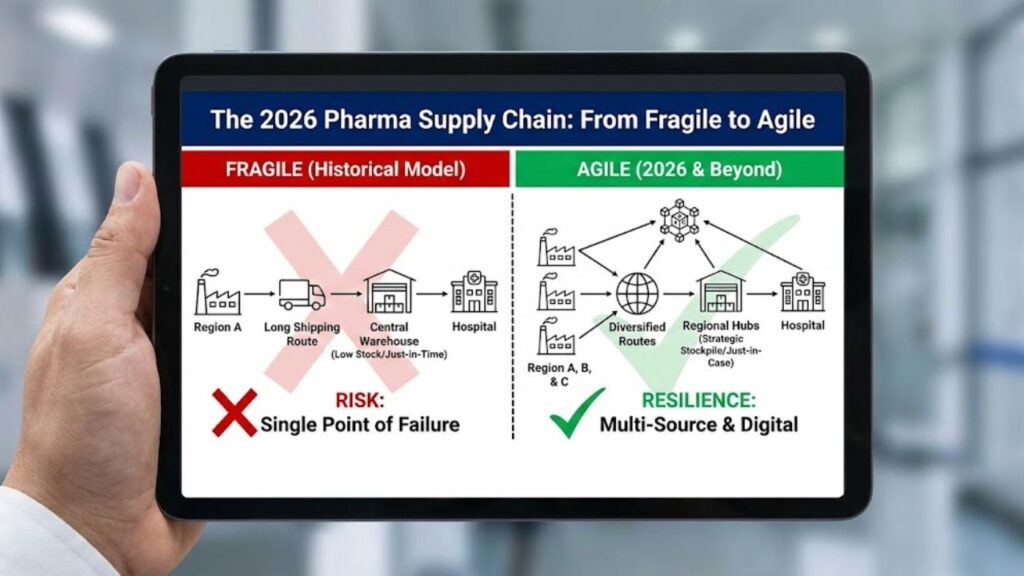
Supply Chain Resilience
Post-pandemic lessons have stuck. We are helping clients move from “Just-in-Time” to “Just-in-Case” supply chain models, ensuring critical raw materials are sourced from diverse, stable regions.
Workforce and Manufacturing Excellence
Implementing these global strategies requires a highly skilled workforce capable of operating advanced manufacturing technologies. As noted by Purdue University’s Pharmaceutical Manufacturing program, the industry faces a skill mismatch as automation and digitization reshape roles. Consultants fill this immediate expertise gap, providing the senior-level technical oversight that growing companies often lack in-house.
The Future: Innovation Meets Compliance
The future of pharma is not just about new drugs; it is about new ways of working. Whether it is navigating the FDA’s flexible requirements for cell and gene therapies or leveraging AI for toxicological prediction, the path forward requires a blend of daring innovation and rigid compliance.
The TLDR on Drug Development Consulting in 2026: You cannot be an expert in everything. The complexity of modern drug delivery, the nuance of pediatric regulations, and the logistics of global trials require a team of specialists.
Looking to consult further on any questions related to your pharma needs?