In many industries, a manufacturing defect means a returned product and a frustrated customer. In the pharmaceutical industry, a defect can mean the difference between health and illness, or even life and death.
Because the stakes are incredibly high, the mechanisms ensuring that every tablet, vial, and injection meets rigorous standards must be infallible. This mechanism is Quality Control.
Quality Control (QC) is the operational backbone of pharmaceutical manufacturing. It is the scientific process of testing, measuring, and verifying that a drug product meets its pre-defined specifications for identity, strength, quality, and purity. While often confused with broader quality management concepts, QC is the “boots on the ground” aspect of quality—the laboratory-based analytical work that acts as the final gatekeeper before a product reaches a patient.
This guide explores the intricate world of pharma QC, distinguishing it from QA, outlining its essential tools and types, and reviewing the stringent guidelines that govern its execution.
QA vs QC in the pharmaceutical industry: Understanding the Difference
The distinction between Quality Assurance (QA) and Quality Control (QC) is fundamental to understanding how the industry manages risk in the world of pharma consulting. While they are symbiotic and often housed under the same corporate umbrella, their functions are distinct. And with the industry growing in many ways, it is more important than ever to master the nuances here.
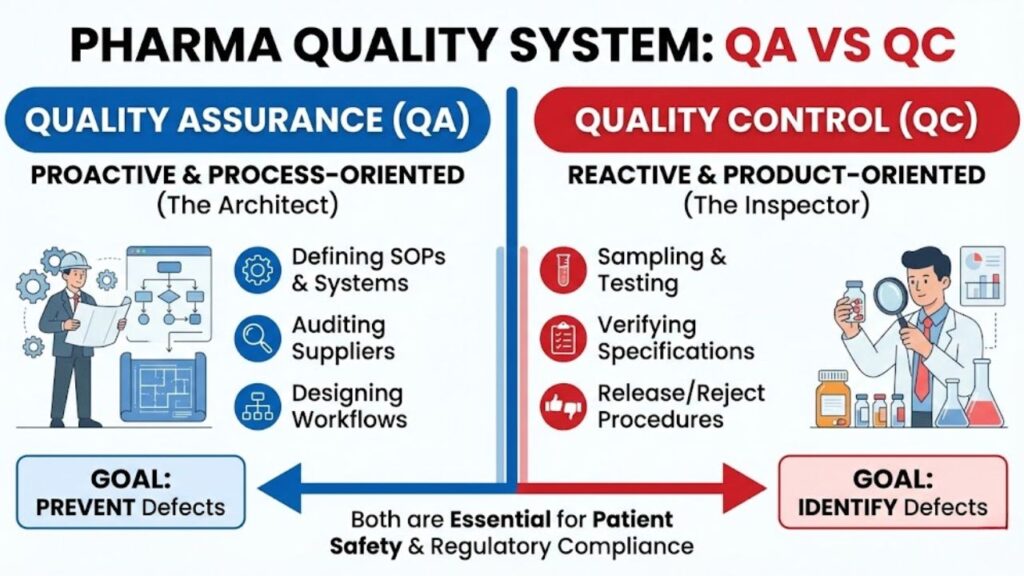
QA vs QC in the pharmaceutical industry can best be understood as the difference between process orientation and product orientation.
Quality Assurance (QA) is proactive. It is the sum of all organized arrangements made with the object of ensuring that medicinal products are of the quality required for their intended use. QA sets up the systems, writes the Standard Operating Procedures (SOPs), audits the suppliers, and designs the manufacturing workflows to prevent errors from occurring in the first place. It is the “architect” designed to build quality into the product from design to delivery.
Quality Control (QC), conversely, is reactive. It is focused on identifying defects in the actual products produced. QC is responsible for sampling, specifications, and testing, as well as the organization, documentation, and release procedures which ensure that the necessary and relevant tests are actually carried out. QC is the “inspector” that tests the final build to ensure it matches the blueprints perfectly. A robust pharmaceutical quality system requires both a strong architect (QA) and a rigorous inspector (QC).
Quality Control (QC) in Pharmaceutical Manufacturing: The Front Line of Safety
Quality Control (QC) in pharmaceutical manufacturing is not merely the final step before shipping. It is an integrated process that runs parallel to the entire production lifecycle. A robust QC unit is independent of production, holding the authority to reject raw materials, halt manufacturing lines, or quarantine finished batches if data suggests a deviation from specifications.
The execution of quality control in pharmaceutical manufacturing involves a sophisticated array of analytical chemistry, microbiology, and physical testing. QC laboratories must be equipped with state-of-the-art instrumentation—such as High-Performance Liquid Chromatography (HPLC), gas chromatography, and mass spectrometry—to detect impurities at microscopic levels. Furthermore, QC is responsible for stability testing, placing products under various conditions of heat and humidity over time to determine their shelf life and ensure they remain effective until their expiration date.
Beyond Testing: What are the 4 types of QC?
To ensure comprehensive coverage, QC activities are generally categorized into four distinct phases spanning the production cycle. When asking, “What are the 4 types of QC?” in the context of pharma, they typically refer to these critical control points:
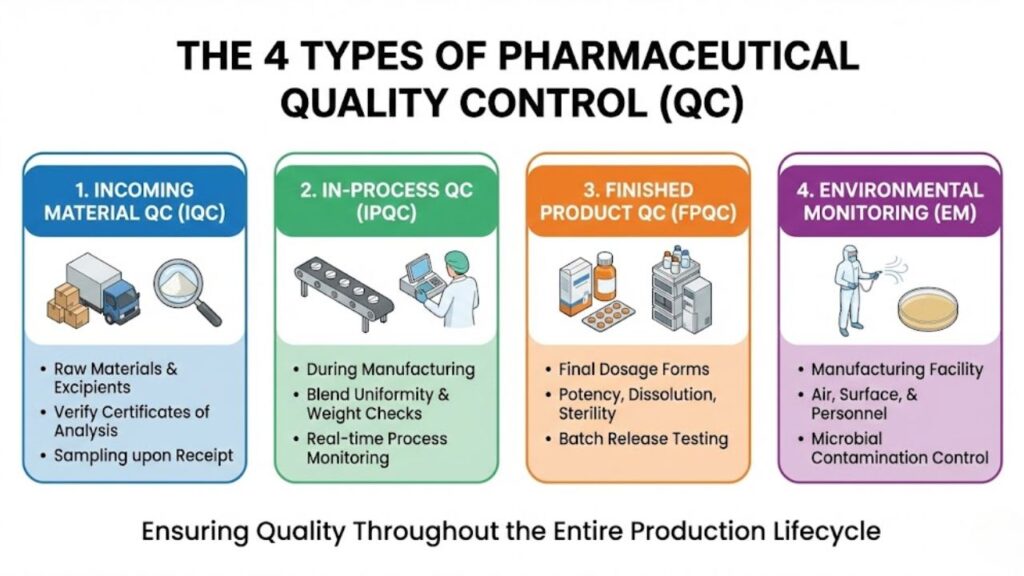
- Incoming Material Quality Control (IQC): Great drugs cannot be made from poor ingredients. IQC involves sampling and testing Active Pharmaceutical Ingredients (APIs), excipients (fillers, binders), and packaging materials immediately upon receipt. These materials must match their Certificate of Analysis (CoA) and meet internal specifications before being released into the warehouse for use in manufacturing.
- In-Process Quality Control (IPQC): Testing shouldn’t wait until the end. IPQC involves checks during the manufacturing process itself. This might include checking the blend uniformity of a powder before it is compressed into a tablet, measuring the pH of a solution during mixing, or verifying the torque of a bottle cap on the assembly line. These checks ensure the process remains in a state of control.
- Finished Product Quality Control (FPQC): This is the final battery of tests on the completed, packaged product. It verifies that the batch meets all release specifications for potency, dissolution (how fast it breaks down in the body), sterility (for injectables), and physical appearance. Only after passing FPQC can a batch be certified by a Qualified Person (QP) for market release.
- Environmental Monitoring (EM): Especially critical in sterile manufacturing, EM is the ongoing QC of the manufacturing facility itself. It involves testing air particulates, surfaces, and personnel gowning for microbial contamination to ensure the “cleanroom” environment is not compromising the product.
The Analytical Toolkit: What are the 7 QC rules?
While pharma relies heavily on advanced chemistry, it also utilizes established statistical tools to monitor process stability. When considering process variations, one might ask, “What are the 7 QC rules?” These refer to the “7 Basic Tools of Quality,” originally popularized by quality guru Kaoru Ishikawa. In pharma, these tools help move QC from simple pass/fail testing to understanding why a process might be drifting.
They include:
- Cause-and-Effect Diagram (Ishikawa/Fishbone): Used in investigations to identify root causes of a lab error or manufacturing deviation.
- Check Sheet: Structured forms for collecting data in real-time on the manufacturing floor.
- Control Chart: Vital for monitoring process stability over time, showing if a critical parameter (like tablet weight) is staying within statistical control limits.
- Histogram: A bar graph showing frequency distributions, useful for seeing if process data is centered around the target specification.
- Pareto Chart: Based on the 80/20 rule, helping QC teams prioritize which types of defects are causing the most issues.
- Scatter Diagram: Used to analyze relationships between two variables, such as mixer speed vs. blend uniformity.
- Stratification (Flow Chart/Run Chart): Separating data from different sources (e.g., different shifts or machines) to find patterns.
Navigating the Regulatory Landscape: Quality control guidelines
Quality Control does not operate based on internal whim; it is tightly governed by regulatory statutes known as current Good Manufacturing Practices (cGMP). These quality control guidelines are enforced by agencies like the FDA (USA), EMA (Europe), and PMDA (Japan).
The FDA’s cGMP regulations specifically delineate the responsibilities of the Quality Control unit. For instance, 21 CFR Part 211 outlines requirements for laboratory controls, testing procedures, and the independence of the quality unit. Failure to adhere to these regulations results in FDA Form 483s, warning letters, or consent decrees.
Furthermore, global harmonization efforts, led by the International Council for Harmonisation (ICH), provide detailed technical guidelines. The ICH Q7 guideline, for example, provides specific GMP guidance for Active Pharmaceutical Ingredients, heavily emphasizing QC’s role in validation and change control. These federal and international guidelines form the non-negotiable framework within which pharma QC must operate.
The paramount importance of quality control in the pharmaceutical industry
The importance of quality control in the pharmaceutical industry cannot be overstated. It is the firewall that protects patients from sub-potent, contaminated, or dangerous medicines. A failure in QC doesn’t just cost money; it damages public trust in science and medicine.
Beyond patient safety, robust QC is essential for business continuity. The academic and scientific rigor required for drug development must be matched by equal rigor in commercial manufacturing. Institutions like Purdue University’s Department of Industrial and Physical Pharmacy emphasize that understanding the physical and chemical properties of drugs through rigorous analysis is foundational to ensuring consistent manufacturing quality.
Furthermore, the economic implications of poor quality are staggering. Recall costs, litigation, and regulatory shutdowns can bankrupt a company. Research emanating from centers like Johns Hopkins Bloomberg School of Public Health often highlights the critical role of regulatory oversight and rigorous testing standards in preventing adverse drug events that burden healthcare systems.
Ultimately, Quality Control is the translation of the R&D promise into a reproducible commercial reality. As the industry moves toward more complex biologics and personalized medicines, the reliance on highly sophisticated analytical chemistry—a discipline championed by programs at universities like the University of Kansas Department of Pharmaceutical Chemistry—will only grow.
Quality Control is not merely a department that performs tests; it is an ethos of scientific integrity integrated into every step of pharmaceutical production. It is the industry’s commitment to the patient that the medicine they take today is exactly the same as the medicine that was proven safe and effective in clinical trials.
Every pharmaceutical challenge is unique. That’s why we start with a no-obligation consultation to understand your specific needs—whether it’s accelerating timelines, closing compliance gaps, or optimizing manufacturing operations.