Intro to Pharma Consulting: The Guide Behind the Science
When most people think of the pharmaceutical industry, they picture scientists in cleanrooms developing breakthrough therapies or robotic manufacturing lines filling millions of vials. These are the visible achievements of drug development. However, behind every approved therapy, from the first investigational molecule tested in clinical trials to the prescription dispensed at a pharmacy, lies a complex architecture of specialized technical expertise that most companies cannot sustain in-house across all disciplines.
This is the world of pharma consulting.

At its core, pharma consulting delivers specialized expertise that bridges critical capability gaps throughout the drug development and commercialization lifecycle. For a biotech startup preparing its first Investigational New Drug (IND) submission, consultants assemble compliant Chemistry, Manufacturing, and Controls (CMC) documentation, design Good Laboratory Practices (GLP) compliant toxicology studies, and prepare pre-IND meeting materials that position the submission for 30-day FDA clearance. For a multinational corporation integrating a recent acquisition, consultants consolidate duplicated supply chains, align quality management systems across manufacturing sites, and migrate IT infrastructure to maintain regulatory compliance while capturing cost synergies.
The industry is vast and complex. According to the U.S. Bureau of Labor Statistics, the medical and pharmaceutical sciences sector is projected to grow significantly, driven by an aging population and the need for life-saving treatments. However, the path to market is fraught with hurdles. A study by the Congressional Budget Office estimates that the average cost to develop a new drug often exceeds $1 billion, with a timeline stretching over a decade.
For industry veterans and newcomers alike, understanding who these consultants are and what they do is essential. This guide peels back the curtain on the experts who help turn chemical compounds into cures.
The “Big Picture” Architects: Strategy & Management
A drug is a scientific breakthrough, but it is also a business asset. If a pharmaceutical company cannot define the pathway to approval or how to organize its teams effectively, even the most promising molecule will fail to reach patients. This is where the architects come in.
Pharma Strategy Consulting
Strategic consultants design the regulatory roadmap from preclinical candidate through IND submission to Biologic License Application (BLA) or New Drug Application (NDA). Consultants identify critical-path activities and phase-appropriate data requirements that support target development milestones while avoiding costly clinical holds.
- IND Readiness Planning: Pharma strategy consultants conduct gap assessments across nonclinical, CMC, and clinical domains to determine which GLP toxicology studies, manufacturing scale-up activities, and analytical validations must be completed. They create integrated timelines aligning preclinical work with pre-IND meetings, FDA’s 30-day review window, and clinical milestones through NDA/BLA submission.
- Regulatory Pathway Optimization: Strategic consultants identify expedited designation opportunities (Fast Track, Breakthrough Therapy, RMAT, PRIME) and prepare briefing packages that address CMC strategies, dose justifications, and clinical protocols, positioning sponsors to receive actionable FDA feedback that accelerates IND clearance.
- Cross-Functional Integration: Consultants design processes that satisfy GMP standards while supporting registration-batch production, establish comparability protocols for process changes, and create risk mitigation plans that address potential safety signals before they trigger clinical holds.
Pharma Management Consulting
While strategists look outward at the market, management consultants look inward at the organization. They answer the “How?” questions.
-
- Operational Efficiency: How do we speed up clinical trials? How do we integrate two different corporate cultures after a merger?
-
- Change Management: Implementing new digital tools or restructuring R&D departments often faces internal resistance. Management consultants smooth these transitions using frameworks validated by decades of organizational psychology research.
The Builders: Engineering the Process
Once the regulatory strategy is established, the physical reality of drug manufacturing must be engineered. Can the laboratory process scale to commercial production? Can it satisfy cGMP requirements and withstand FDA inspection?
This is the domain of pharma engineering consultants.
These specialists translate process chemistry into GMP-compliant manufacturing systems. These consultants design integrated facility ecosystems where cleanroom classification, environmental controls, and equipment qualification converge to support sterile production. They specify process equipment, bioreactors, filtration trains, chromatography skids, filling lines, with User Requirement Specifications (URS) that define operational parameters, cleaning validation requirements, and automation integration necessary for batch record integrity and 21 CFR Part 11 compliance.
The Critical Role of GMP
The guiding bible for this sector is Good Manufacturing Practice (GMP). This is not just a suggestion; it is a legal requirement enforced by agencies like the FDA.
-
- Beginner Concept: The factory must be clean and run correctly.
-
- Veteran Concept: Engineering consultants focus on “Sterility Assurance Levels” (SAL) and validate complex bioreactor trains. They ensure that every pipe, valve, and sensor is “qualified”—meaning it is proven to work exactly as intended, every single time.
For a deeper dive into the regulatory standards that engineering consultants must navigate, the FDA’s guide on Current Good Manufacturing Practice (CGMP) provides the federal statutes that dictate how facilities must be designed and monitored.
Tech Transfer
One of the most delicate moments in a drug’s life is “tech transfer”—moving the recipe from a small lab beaker to a 2,000-liter reactor. Engineering consultants specialize in scaling up these processes, using fluid dynamics and thermodynamics to ensure the chemical reaction happens exactly the same way at scale as it did in the lab.
The Backbone: Supply Chain & Procurement
A drug is useless if you cannot get the raw materials to make it or the finished product to the hospital. While scientists work on the molecular level, supply chain consultants work on the global level, orchestrating a complex web of logistics that keeps the industry moving.
Pharma Procurement Consulting
This is the “upstream” battle: securing the ingredients.
-
- The Sourcing Challenge: Most Active Pharmaceutical Ingredients (APIs) are manufactured in specific regions (often India and China). Consultants help companies diversify their sourcing to avoid a single point of failure, a lesson learned the hard way during recent global disruptions.
-
- Negotiation & Risk: It isn’t just about buying cheap; it’s about buying safe. Procurement consultants vet vendors for geopolitical stability and ethical labor practices, ensuring the company doesn’t wake up to a scandal or a shortage.
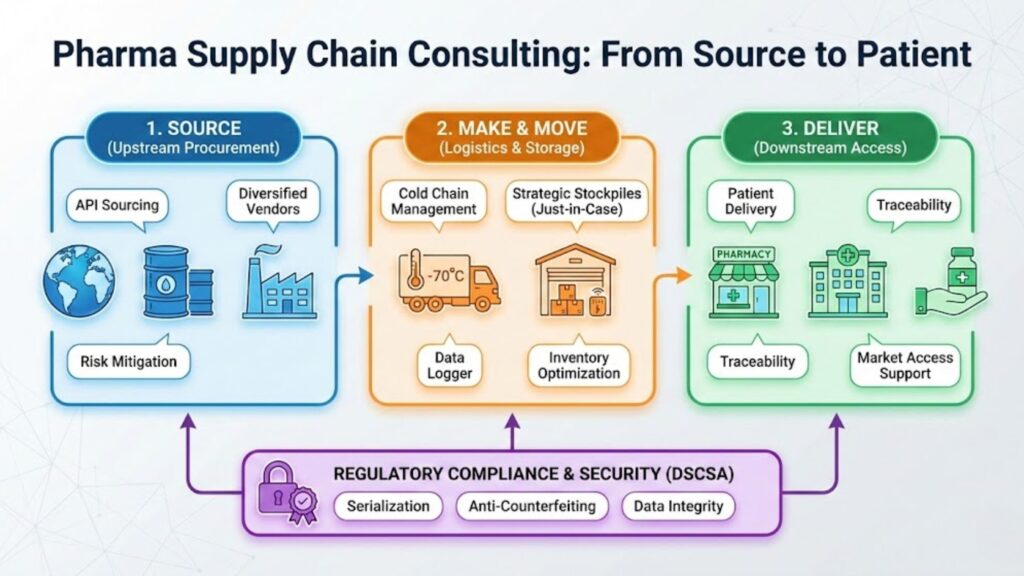
Pharma Supply Chain Consulting
This is the “downstream” journey: getting the box to the patient.
-
- Cold Chain Mastery: Biologics and vaccines often require strict temperature controls (e.g., -70°C). Consultants design “cold chain” logistics networks that use data loggers and specialized packaging to ensure a life-saving vaccine doesn’t turn into a useless vial of warm water during transit.
-
- The “Just-in-Case” Model: For decades, the industry relied on “Just-in-Time” inventory to save money. Post-pandemic, consultants are helping firms pivot to “Just-in-Case” models, building strategic stockpiles to weather future storms.
Regulatory Spotlight: A major focus for supply chain consultants today is the Drug Supply Chain Security Act (DSCSA). This FDA mandate requires an electronic, interoperable system to identify and trace prescription drugs. It’s about preventing counterfeits. You can read the specific requirements for tracing and serialization directly on the FDA’s DSCSA implementation page.
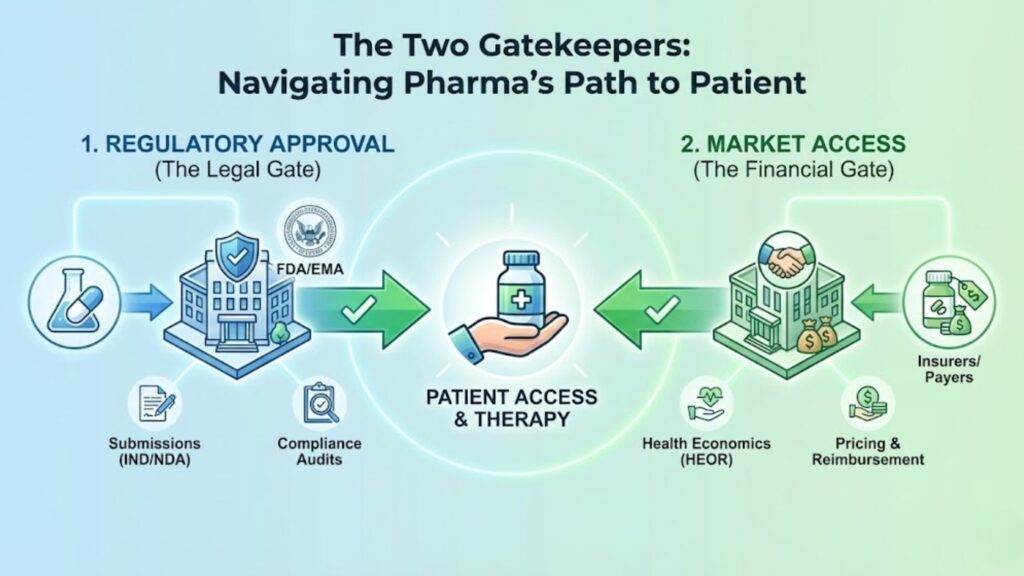
The Gatekeepers: Regulatory & Market Access
There are two massive hurdles every drug must clear before a single dollar is made. First, the government must say it is legal (Regulatory). Second, the insurers must agree to pay for it (Market Access).
Pharma Regulatory Consultants
These are the navigators of bureaucracy. They translate scientific data into the rigid formats required by agencies like the FDA (USA) or EMA (Europe).
-
- The Submissions: They handle the massive paperwork loads—Investigational New Drug (IND) applications to start trials, and New Drug Applications (NDA) to sell the product.
-
- Crisis Management: If an FDA auditor finds rust in a mixing tank, regulatory consultants are the “fixers” who write the remediation plan to keep the factory open.
-
- Learn the Process: The path from lab to approval is rigid. The FDA outlines this multi-step journey clearly in their Drug Development Process guide.
Market Access Consulting Pharma
Approval does not guarantee profit. If a drug costs $50,000 but insurers refuse to cover it, the drug effectively doesn’t exist for the patient.
-
- Value Proposition: These consultants use Health Economics and Outcomes Research (HEOR) to prove that a drug isn’t just effective, but cost-effective. Does it save money by preventing hospitalizations?
-
- Reimbursement Strategy: They negotiate with public payers (like Medicare) and private insurers. For a look at how coverage decisions are actually made at the federal level, review the CMS National Coverage Determination process, which dictates what Medicare will and will not pay for.
The Future: Innovation & Digital Transformation
Pharma has historically been slow to modernize—mostly due to the fear that “new” equals “risky.” Consultants are the accelerators who show companies how to innovate without breaking compliance.
How Consulting Firms Help Pharma Companies Adopt New Technologies
-
- AI in Discovery: Instead of testing 10,000 compounds physically, AI models can simulate them virtually, identifying the most promising candidates in weeks rather than years.
-
- Digital Twins: Engineering consultants can now build a “digital twin” of a factory, a virtual replica that runs simulations to predict breakdowns before they happen in the real world.
Expert Resource: The FDA is actively encouraging this shift. Their Digital Health Center of Excellence provides resources and regulatory frameworks for companies looking to integrate AI and digital tools into medical products.
The Ecosystem: Who Are the Players?
The pharma consulting landscape is not a monolith; it is a tiered ecosystem. Knowing which type of firm to hire is half the battle. If you hire a strategy giant to fix a leaky pipe in a factory, you are overpaying. If you hire a boutique engineer to design a global M&A strategy, you are under-thinking.
The Strategic Giants
These are the firms you call for the “Boardroom Problems.”
-
- The Big Three (MBB): McKinsey, BCG, Bain. They define the industry standard for high-level strategy.
-
- The Role: They handle mergers, acquisitions, and organizational restructuring.
The Data & Clinical Specialists
-
- The Players: IQVIA, Parexel, Syneos Health.
-
- The Role: They don’t just advise; they execute clinical trials and crunch the massive datasets required for FDA submission. They own the “patient data” niche.
The Boutique Experts (The “DES Pharma” Tier)
-
- The Role: specialized, agile, and often staffed by industry veterans rather than generalist MBAs.
-
- Why they exist: When you need a specific problem solved—like a supply chain bottleneck for a gene therapy or a regulatory audit for a new facility—you don’t need a generalist; you need a surgeon.
Note: This is where DES Pharma operates. We don’t try to be everything to everyone. We are the precise tool for specific, high-value technical and operational challenges.
The TLDR on Pharma Consulting in 2026 and Beyond:
Pharma consulting is more than just an external brain trust. It is an insurance policy against the complexity of modern science.
Whether you are navigating the murky waters of pharma supply chain consulting, trying to digitize a 20-year-old factory, or figuring out the pricing strategy for a rare disease cure, you cannot do it alone. The regulations are too strict, and the competition is too fast.
At DES Pharma, we believe that the best consultants don’t just leave you with a PowerPoint deck; they leave you with a working solution.
Ready to Turn Complexity Into Clarity?
Whether you’re a biotech startup preparing your first IND submission, a multinational corporation navigating post-merger integration, or a pharmaceutical manufacturer scaling up to commercial production, the right expertise makes the difference between costly delays and regulatory success.
At DES Pharma, we don’t operate as generalist advisors—we’re precision problem-solvers. Our team of seasoned technical operations experts brings 25+ years of hands-on experience across process development, CMC regulatory strategy, GMP engineering, manufacturing science and technology, and supply chain optimization. We work alongside your team to deliver actionable solutions, not just presentations.
What We Can Help You With:
Regulatory Strategy & IND Readiness
Gap assessments, pre-IND meeting preparation, CMC documentation, and expedited pathway applications that position your submission for first-time FDA clearance.
Process Development & Scale-Up
Technology transfer, manufacturing process characterization, equipment qualification, and GMP-compliant facility design that ensures your lab-scale process translates to commercial production.
Supply Chain & Procurement Optimization
Supplier diversification strategies, cold chain logistics design, DSCSA compliance implementation, and risk mitigation planning that protects continuity of supply.
Quality & Compliance
cGMP audit remediation, validation protocol development, 483 response support, and quality management system alignment across manufacturing sites.
Get Your Customized Project Quote
Every pharmaceutical challenge is unique. That’s why we start with a no-obligation consultation to understand your specific needs—whether it’s accelerating timelines, closing compliance gaps, or optimizing manufacturing operations.